Экология и Биология Рыбного Филина
ПРОЦЕС ПЕРЕВОДА ИДЁТ...
Грубый и неполный перебод статья
Slaght, J.C., and S.G. Surmach. 2008. Biology and conservation of Blakiston's fish owls in Russia: a review of the primary literature and assessment of the secondary literature. Journal of Raptor Research 42:29-37.
1. Распространение
2. Популация
3. Место обитания, Размножение, Поведение
4. Кормовая база и Охота
5. Вокализация
6. Угрозы
Рыбный филин (Ketupa blakistoni) обитает только в северо-восточнй Азии—в Японии, на Дальнем Востоке России, и в Китае. Существуют два подвида--островной (K. b. blakistoni), которой обитает на островах Хоккайдо, Кунашир, и Шихотан (Дыхан и Кислейко 1988, Brazil and Yamamoto 1989, Takenaka 1998, Берзан 2005), и материкой (K. b. doerriesi), который живёт от Приморьского края до Магадана в России, и наверно в Китае (Сурмач 1998). Возможно, тоже существует в Северной Корее, но нет достоверных встреч (W. Duckworth pers. comm.).
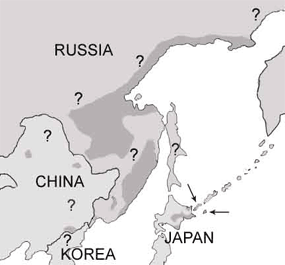
Из <60 размножающих пар (всего 150-205 птиц), которые составляют островной подвид, 30-35 пар (80-120 оссобей) живут на о. Хоккайдо в Японии (Brazil and Yamamoto 1989, Takenaka 1998), и осталные 20-25 пар (70-85 оссобей) живут воосновном на о. Кунашир (Дыхан и Кислейко 1988, Берзан 2005). Были свежые встречи на о. Шихотан, но ражмножение там не потвердили (Григорьев 2005).
Материкной подвид труднее числить, из-за огромное пространство ареала, и мало или совсем нет учётов в этих местах. Сурмач (1998) писал, что популяция Уссурийского края (считая Приморский краи и Хабаровского края юг от реки Амур) около 100-130 пар судя по его учёты. С экстраполяцием всему ареалу рыбного филина, могут быт около 800 пар (S. Surmach, в Collar 2001).
Сурмач (2006) обнаружил один пар каждый 3.8 км реки на р. Самарга в северном Приморьу--возможно самая высокая плотность данного вида на свете. Другие авторы предпологали, что рыбный филин занимается 6-12 rкм реки (Перерва 1984, Voous 1988, Hume 1991, Collar et al. 1994, del Hoyo et al. 1999, König et al. 1999).
3. Место обитания, Размножение, Поведение
Рыбный филин требует крупных, старых деревьев для гнездования, и незамёрших рек, богатые рыбами (Яковлев 1929, Воробёв 1954, Шибнев 1963, Пукинский 1973, Дыхан и Кислейко 1988, Дугинцов и Терёшкин 2005). Из 23 известных гнёзд в литературе, 8 явдяется ильм (Ulmus sp.), 5--топол (Populus maximowiczii), 4--ива ( Salix sp.), 2--чозенья (Chosenia arbutifolia ), 2--дуб (Quercus mongolica), 1--Sorbus sp., и 1--берёза (Betula ermanii). Nest types are cavity nests ( N= 12), broken-top nests ( N= 7), tree fork nests ( N= 1), and unspecified ( N= 3). Высота гнёзды 2-18 m (Нечаев и Куренков 1986, Дыхан и Кислейко 1988, Воронов и Здориков 1988, Takenaka 1998, Пукинский 1973, 1993, Дугинцов и Терёшкин 2005, Елсуков 2005, Сурмач неопубликован).
Кроме старых, дуплистих деревев, во время зимы присуствие открытая вода очень важная для рыбного филина (Яковлев 1929, Спангенберг 1948, Сурмач 1998). Незамёрщая вода находется только там, где течение достаточо быстрая, или где тёплый родник впадает в реку (Сурмач 1998). Дугинцов и Терёшкин (2005) сообшили, что такие места наидётся каждый 5-7 км на р. Селемжа. Сурмач (1998) писал, что slower-moving streams are more important to Blakiston's fish owls than the main river channels, and that, given sufficient prey, openings as small as a few m 2 are sufficient to sustain a pair of resident owls through the winter.
Blakiston's fish owls can form pair bonds as early as their second yr, and reach sexual maturity by age 3 (Pukinskii 1973). It is not clear what dictates breeding attempts, as the species does not necessarily breed every yr (Pukinskii 1973, Berzan 2000, Dugintsov and Teryoshkin 2005, S. Surmach unpubl. data). Breeding is likely tied to environmental and other factors not yet identified. Courtship occurs from January-February, with a clutch of one or two eggs laid in March (Nechaev 1969, Pukinskii 1973, Dykhan and Kisleiko 1988, Voronov and Zdorikov 1988). The female incubates the clutch while the male brings food to the nest (Brazil 1985, S. Surmach, pers. obs.) Once chicks hatch, the female continues to warm them during the day, but joins the male to bring food to the nest at night (J. Slaght, pers. obs.). Young fledge up to 50 d post-hatching (Pukinskii 1993). Data on breeding success are scant: on Kunashir Island during a 6 yr period breeding success was 24%; with six fledglings resulting from 25 eggs (Berzan 2000). Juveniles remain on their natal territory into their second yr, apparently dispersing as late as July the following yr (Pukinskii 1973). This unusually long pre-dispersal period may be why Shibnev (1963) interprets several full-grown fish owls in a single tree in April as evidence of gregarious behavior, and is repeated as such by Voous (1988). However, Pukinskii (1973) considers this a misunderstanding of fish owl biology.
An observation by Pukinskii (1973) of congregations of five-six birds at open-water areas is also misinterpreted by several secondary sources (Voous 1988, Hume 1991) as evidence of gregarious behavior. The original text clearly identifies such congregations as rare events in unseasonably cold winters, when regular foraging habitat is presumably ice-covered and therefore inaccessible.
Both Pukinskii (1973) and Mikhailov and Shibnev (1998) stated that fish owls are non-migratory. Although Pukinskii (1973) dismissed a description by Shibnev (1963) of apparent fish owl migration in the Bikin River basin in winter, several secondary sources have cited it (Voous 1988, König et. al. 1999). At present however, there are few data to verify either assertion, as fish owl seasonal movements are poorly understood. Although long-distance migration is unlikely (Pukinskii 1973, Mikhailov and Shibnev 1998, S. Surmach unpubl. data), short distance movements, such as seasonal shifts in home range, may occur and should be investigated.
Several sources link Blakiston's fish owl distribution to rivers rich with salmonid fish (Pukinskii 1973, Dykhan and Kisleiko 1988), but it appears that fish owls can persist on smaller prey species as well (Surmach 1998). Driven by seasonal and spatial availability, fish owl prey base is actually quite diverse: in addition to fish, direct observation or examination of prey remains shows predation of a variety of waterfowl species, small mammals, and amphibians (Vorobev 1954, Pukinskii 1973, Dykhan and Kisleiko 1988, Voronov and Zdorikov 1988, Dugintsov and Teryoshkin 2005). Reliance on certain prey species is seasonal: in spring, frogs ( Rana sp.) are particularly important and taken in great abundance (Pukinskii 1973). Interestingly, although many secondary sources (Fogden 1973, 1992, Flint et al. 1984, König et al. 1999, Collar 2001) list crayfish as an important food source, the most recent documentation of crayfish predation on the mainland is by Pukinskii (1973), with the only other report from Yakovlev (1929), although Voronov and Zdorikov (1988) describe finding crustacean remains in fish owl pellets on Kunashir Island. A similar report by Pererva (1984) erroneously cites Dementev (1951) as a reference, although this information is not found in that publication. Anecdotal reports of crayfish population declines in Primorye over the last several decades may account for their apparent disappearance from fish owl diet on the mainland.
The most commonly described hunting technique used by fish owls is dropping on prey in shallow water, often from a low, nearby perch, such as a snag or rock, or in winter simply from the ice edge (Yakovlev 1929, Pukinskii 1973, Dykhan and Kisleiko 1988, Voronov and Zdorikov 1988, Dugintsov and Teryoshkin 2005). Upon identifying prey, fish owls either drop directly into the shallow water or sail a short distance (Yakovlev 1929, Pukinskii 1973, Dykhan and Kisleiko 1988, Voronov and Zdorikov 1988). Fish owls generally take frogs and other smaller prey directly back to a survey perch or another roost to be consumed. Larger prey such as fish and waterfowl are partially consumed on the riverbank or ice edge before being taken to a habitual roost to be finished (Yakovlev 1929, Pukinksii 1973, Voronov and Zdorikov 1988, Dugintsov and Teryoshkin 2005).
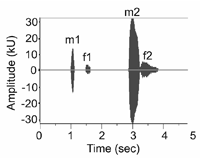
Пукинский (1974) описал некоторые вокализации рыбного филина, наверно самая интересная из них является дуэт. Структура дуэта материкого подвида отличается от дуэта островного подвида. На материке, дуэт составляет 4 ноты, где самец издаёт первые и третые ноты, а самка--вторые и четвёртые ноты (Рис. 2 и 3). В противположности, дуэт островного подвида составляет лишь 3 ноты--самец сразу издаёт две ноты и самка отвечает одной нотой (Brazil and Yamamoto 1989).

Although other sources of mortality may remain undetected due to lack of study, most known Blakiston's fish owl mortality is due to contact with humans (all but two records; Pukinskii 1993, Yelsukov 2005). Of 12 cases of fish owl mortality known to Yelsukov (2005), all but three birds were shot by hunters. Surmach (1998) reports 10 cases of fish owls shot wantonly, and two birds shot for commercial gain (i.e., sale of live-mount specimens). In winter fish owls are accidentally caught in furbearer snare lines (Vorobev 1954, Dykhan and Kisleiko 1988, Averin and Antonov 2005, Yelsukov 2005). Surmach (1998) believes fish owls can survive 3-5 d in such snares, and are often healthy enough to be released once found, but fish owls were killed by the trapper in 41 of 48 known cases of trapping. Education of local populations may therefore be a key conservation tool (Pererva 1984, Surmach 1998).
Direct hunting of fish owls, although not common, should also be considered a threat. The native Udege peoples of the Bikin and Samarga river basins target the owls in winter as a food source (Vorobev 1954, Mikhailov and Shibnev 1998). Meise (1933) reports similar findings from Manchuria . Spangenberg (1965) describes encounters along the Iman River in 1938-39 with Udege who use dried fish owl wings as fans to dissipate biting insect swarms. Recent interviews with Udege in the Samarga River basin indicate fish owl hunting is not as widespread as it once may have been (J. Slaght pers. obs.).
Logging is a considerable threat to Blakiston's fish owl conservation. Although in theory Russian law protects riparian areas from harvest, as logging within 5 km of either bank of major waterways is illegal (Surmach 1998), riparian areas are still being impacted by logging either directly or indirectly. First, the law is not always enforced in remote areas of the Russian Far East, and loopholes in the law are often exploited (Newell and Lebedev 2000). Riparian areas are also impacted indirectly by the creation of roads to facilitate resource extraction (Slaght 2005). Old-growth Japanese poplar—a favored fish owl nest tree species—is chosen by loggers to create makeshift bridges across waterways (J. Slaght pers. obs.). Ash and Mongolian oak, also important fish owl nest tree species, are valued in the timber industry and removed illegally in high volume (Newell and Lebedev 2000). Furthermore, logging roads often remain accessible after an area has been harvested, which facilitates illegal logging in riparian areas (Vandergert and Newell 2003). Following an example from Japan where artificial nest boxes were used to restore nesting opportunities in logged forest (Brazil 1985), nest boxes were constructed on Kunashir Island after natural senescence of nest trees was found to cause nest tree abandonment (Berzan 2000, Grigorev 2005). In both cases, the owls readily utilized the artificial nests and successfully fledged young.
The notion that Blakiston's fish owls are intolerant of human encroachment is prevalent in the secondary literature (i.e. Voous 1988, Hume 1991, del Hoyo et al. 1999, Collar 2001). However, this may not be a wholly applicable generalization (Surmach 1998). First, not all of these reports are credible: Collar (2001) cites Vaskovskii (1956) when noting fish owl displacement in Magadan due to increased human activity. However the original text indicates the opposite, that fish owls are observed there with some regularity. In fact, there are numerous records of fish owls found close to human settlements and even nesting in proximity to villages (Bergman 1935, Gizenko 1955, Pukinskii 1973, Tarkhov and Potapov 1986, Shokhrin 2005, S. Surmach unpubl. data). Furthermore, we know of several successful fish owl nests located near human foot trails, and a pair of owls that nested in a forest selectively logged several decades ago. From a conservation perspective, all of these observations suggest an encouraging level of tolerance and adaptability by the species (Surmach 1998), although more study is needed to evaluate reproductive success and survivorship of individuals living near human settlements.